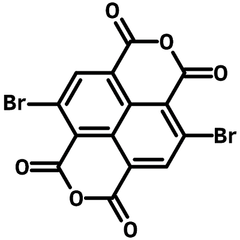
2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic Dianhydride
CAS Number 83204-68-6
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, Monomers
2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic Dianhydride (NTCDA-2Br)
A building block for the synthesis of n-type semiconducting polymers and dyes for polymer solar cells, OFETs and bioimaging applications
2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride (NTCDA-2Br, CAS number 83204-68-6) is a symmetric naphthalene derivative with adjacent carboxylic acids forming anhydride on both ends of the structure. It is a precursor to naphthalene diimides (NDIs) which are used as active layer materials in organic solar cells, field-effect transistors.
2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride possesses aromatic planar conjugated structure and is favourable for good charge transport. Materials composed of naphthalene-1,4,5,8-tetracarboxylic dianhydride (NTDCA) or naphthalene-diimides (NDIs) have an excellent combination of redox, electrical, optical and thermal properties with high moisture and oxygen resistance.
2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride on its own could be used as cathode interlayer materials for efficient electron extraction.
General Information
| CAS Number | 83204-68-6 |
| Chemical Formula | C14H2Br2O6 |
| Full Name | 2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic Dianhydride |
| Molecular Weight | 425.97 g/mol |
| Synonyms |
4,9-Dibromoisochromeno[6,5,4-def]isochromene1,3,6,8-tetraone, NTCDA-2Br |
| Classification / Family |
Naphthalenes, Monomers, Electron transport layer materials |
Chemical Structure
Product Details
| Purity | >98% (GC) |
| Melting Point | 350 °C |
| Appearance | Yellow to dark green powder/crystals |
MSDS Documentation
 2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride MSDS Sheet
2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride MSDS Sheet
Literature and Reviews
-
A Balanced Face-On to Edge-On Texture Ratio in Naphthalene Diimide-Based Polymers with Hybrid Siloxane Chains Directs Highly Efficient Electron Transport, Y. Kim et al., Macromolecules 48 (15), 5179–5187 (2015); DOI: 10.1021/acs.macromol.5b01012.
-
The Effect of Alkyl Spacers on the Mixed Ionic-Electronic Conduction Properties of N-Type Polymers, I. Maria et al., Adv. Funct. Mater., 31, 2008718 (2021); DOI: 10.1002/adfm.202008718.
-
The synthesis of novel core-substituted naphthalene diimides via Suzuki cross-coupling and their properties, S. Bhosale et al., New J. Chem., 33, 2409-2413 (2009); DOI: 10.1039/B9NJ00476A.
-
Rod-like oligomers incorporating 2,6-dialkylamino core-substituted naphthalene diimide as acceptors for organic photovoltaic, R. Fernando et al., Org. Electron., 14 (6), 683-1692 (2013); DOI: 10.1016/j.orgel.2013.03.039.
- Improved Performance of Solution-Processed n‑Type Organic Field-Effect Transistors by Regulating the Intermolecular Interactions and Crystalline Domains on Macroscopic Scale, S. Vasimalla et al., Chem. Mater., 26, 4030−4037 (2014); DOI: 10.1021/cm501780p.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.

![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)