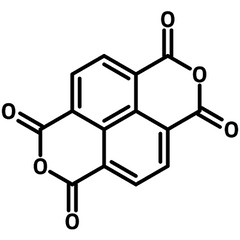
NTCDA - 1,4,5,8-Naphthalenetetracarboxylic dianhydride
CAS Number 81-30-1
Chemistry Building Blocks, Diamines and Dianhydrides, Heterocyclic Building Blocks, Materials, Monomers
NTCDA, Most commonly used as a precursor to naphthalenediimides
Interacts strongly with metal and to create a charge transfer complex with ITO.
NTCDA (CAS number 81-30-1) has a naphthalene core with four carboxylic acids at 1,4,5,8-positions, each adjacent carboxylic acids forms an anhydride.
NTCDA (1,4,5,8-naphtalenetetracarboxylic dianhydride) is aromatic, stable, planar and highly symmetric with unusual electrical properties. Due to the electron deficient nature of the carboxylic groups, NTCDA is well known as a strong electron-accepting molecule with a high electron affinity of 4.0 eV. Studies shows that NTCDA dramatically improve electric conductivity in a co-deposited film of NTCDA and several metals, including indium, magnesium and aluminium. The high electron conductivity of co-deposited NTCDA/In film strongly suggests that a co-deposited NTCDA/In film is an excellent electron transporting layer for OLED and photovoltaic devices.
Electron traps can be created by the introduction of trace amounts of n-type small molecule semiconductor NTCDA into polyethylenimine (PEI), which effectively reduces the leakage current and improves the breakdown strength and energy storage properties of the composite at high temperature. Especially, excellent energy storage performance is achieved in 0.5 vol.% NTCDA/PEI at the high temperatures of 150 and 200 °C.
General Information
| CAS Number | 81-30-1 |
| Chemical Formula | C14H4O6 |
| Full Name | 1,4,5,8-Naphthalenetetracarboxylic dianhydride |
| Molecular Weight | 268.18 g/mol |
| Synonyms | 1,4,5,8-Naphthalenetetracarboxylic dianhydride, Naphthalene-1,4,5,8-tetracarboxylic Dianhydride |
| Classification / Family | Naphthalene, semiconductor synthesis intermediates, Electron donor unit, OLED, OFETs, organic photovoltaics |
Chemical Structure

Product Details
| Purity | >98% (1H NMR) |
| Melting Point | >300 °C (lit.) |
| Appearance | White to brownish powder/crystals |
MSDS Documentation
Literature and Reviews
- Optical transitions in new trends organic materials, S. Pérez-Merchancano et al., Microelectronics J. 39 (3-4), 576-578 (2008); DOI: 10.1016/j.mejo.2007.07.033.
- Superior High-Temperature Energy Density in Molecular Semiconductor/Polymer All-Organic Composites, B. Zhang et al., Adv. Funct. Mater., 2210050 (2022); DOI: 10.1002/adfm.202210050.
- 1,4,5,8-Naphthalenetetracarboxylic dianhydride grafted phthalocyanine macromolecules as an anode material for lithium ion batteries, L. Tao et al., Nanoscale Adv., 3, 3199 (2021); DOI: 10.1039/d1na00115a.
- Efficient p-i-n type organic solar cells incorporating 1,4,5,8-naphthalenetetracarboxylic dianhydride as transparent electron transport material, C. Falkenberg et al., J. Appl. Phys. 104, 034506 (2008); DOI: 10.1063/1.2963992.
- Strong Exciton-Photon Coupling and Exciton Hybridization in a Thermally Evaporated Polycrystalline Film of an Organic Small Molecule, R. Holmes et al., Phys. Rev. Lett., 93 (18); 186404 (2004); DOI:10.1103/PhysRevLett.93.186404.
- Interface state and dipole assisted hole injection improvement with 1,4,5,8,-naphthalene-tetracarboxylic-dianhydride in organic light-emitting devices, P. Jeon et al., Appl. Phys. Lett., 99, 073305 (2011); DOI: 10.1063/1.3628293.
- Organic photovoltaics incorporating electron conducting exciton blocking layers, B. Lassiter et al., Appl. Phys. Lett., 98, 243307 (2011); DOI: 10.1063/1.3598426.
- Spontaneous charge transfer from indium tin oxide to organic molecules for effective hole injection, Y. Koo et al., Appl. Phys. Lett., 94, 153302 (2009); DOI: 10.1063/1.3119860.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
 NTCDA MSDS Sheet
NTCDA MSDS Sheet
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)